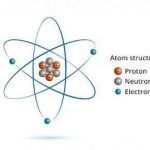
All elements tend to make the electronic configuration of inert gases at their outermost energy level. All inert gases other than helium have 8 electrons at their outermost energy level. When constituting molecule, an element obtains the electronic configuration of inert gases, holding electrons by means of donating, accepting or sharing electrons. This is known as octet rule.